Overview
| Type of Drug | Opiate Analgesic |
| Active Ingredient | Tapentadol Hydrochloride |
| Pain Condition | Acute and Chronic pain |
| Brand names | Nucynta, Palexia |
| Available Strengths | Tapentadol IR ( 50,75,100 mg) Tapentadol ER ( 50, 100, 150, 200,250 mg ) |
| Available As | Immediate Release, Extended Release |
Tapentadol- Key Information
What is Tapentadol
Pain is a global issue with a significant impact on employment, healthcare costs, and quality of life.
Tapentadol is a strong analgesic belonging to the class of medications called opiate analgesics that the European Union has approved for treating wide-ranging pain conditions, including acute or severe pain, including its use in people not responding satisfactorily to other opioid alternatives.
Mechanism and Pharmacokinetics
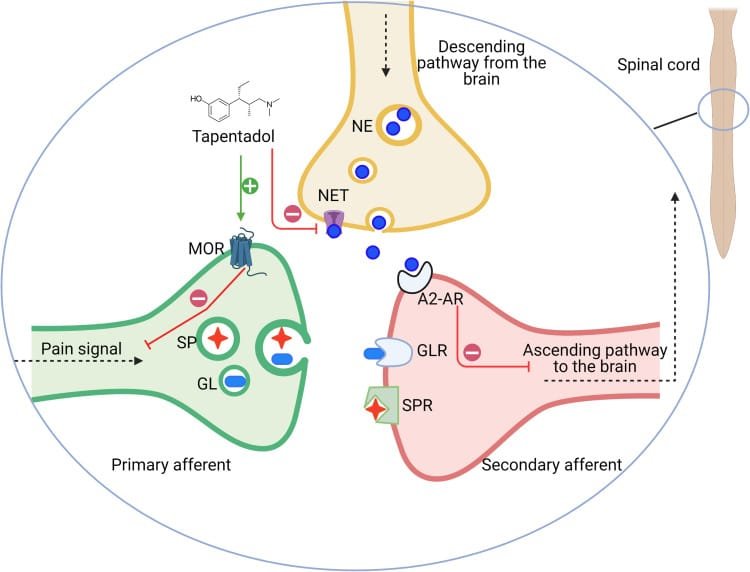
Tapentadol has a dual mechanism of action that works as an agonist of the mu-opioid receptors and as a nor-epinephrine inhibitor, all in a single molecule. Animal studies have demonstrated that Tapentadol shows greater specificity and selectivity towards nor-epinephrine, with a tendency to interrupt ascending pain signals in the spinal cord and enhance descending inhibitory pathways that help it provide satisfactory and reliable pain relief.
Buy Tapentadol in the UK
Tapentadol in the UK is classified as a Class A Controlled Substance under the Misuse of Drugs Regulations and is not readily available over the counter. You will require a prescription to obtain Tapentadol or its refills or need to obtain a prescription to possess the medication.
Usually, pharmacies dealing with opioid analgesics or GP offices with retail counters are a suitable way to procure Tapentadol in the UK. Alternatively, you can order the medication from online retail counters, which will help you get the medicine delivered to your door and ensure deals on the purchase. Some online retail counters even allow online consultation to help you get a prescription to avail of the medication.
Tapentadol and Back Pain
Reports have suggested that approximately 16.9% of adults deal with chronic back pain in the UK, with the problem impacting 30% of young adults aged 18-39.
Because of its unique mechanism of action, Tapentadol Prolonged Release is a suitable option for patients with chronic lower back pain that has neuropathic and nociceptive components in it.
Clinical trials have proven an improvement in the Neck Disability Index Scores from 55.6±18.6 at baseline to 19.7±20.9 at 12 weeks with increased range of motion and significantly decreased mean pain intensity in Tapentadol using patients.
The medication has been recorded to be well tolerated and associated with relevant improvement of movement, functionality, and quality of life.
Tapentadol and Cancer Pain
Oral Tapentadol is considered a good option for people with chronic moderate to severe cancer/tumor pain, especially those intolerant to other opioids or dissatisfied with their analgesic treatment.
Clinical trials conducted on over 343 cancer patients with moderate to severe pain demonstrated that Tapentadol was as good as potent opioids like Oxycodone in reducing pain intensities.
58.7% of the patients treated with Tapentadol reported improved or much-improved pain versus 50.4% in the Oxycodone group. Most importantly, a lower incidence of constipation was recorded in the Tapentadol group.
Tapentadol Approval
Tapentadol has received regulatory approval for acute and chronic pain across various countries. In the United Kingdom, the medication was approved by the MHRA ( Medicine and Healthcare Products Regulatory Agency in 2011. It has further been FDA-approved for the management of moderate to severe pain in adults along with the treatment of diabetic-neuropathy-related pain conditions. In the European Union, Tapentadol Prolonged Release is available under the brand name Palexia. Additionally, both the Scottish and Welsh guidelines have approved prolonged-release Tapentadol for moderate to severe chronic pain that can only be managed by strong opioids other than morphine.
Dosage
Tapentadol Immediate Release is available in strengths of 50, 75, and 100 mg, scheduled every four to six hours.
Tapentadol Extended Release comes in 50, 100, 150, 200, 250 mg scheduled twice daily.
The dosage of Tapentadol is a variable factor depending on
● The type of pain condition, along with the intensity of the problem
● Your tolerance to the medication and its ingredients
● Concomitant use of other medications with Tapentadol
● Treatment plan of the physician
Side Effects
Side effects in Tapentadol are relatively less than that of other opioids. A dry mouth and dyspepsia are some of the chief side effects associated with Tapentadol use.
In a quantitative systematic review carried out to compare Tapentadol and Oxycodone in pain management, the risk of opioid-based adverse reactions in the form of pruritus, nausea, vomiting, obstipation, and somnolence was reduced when Tapentadol was used for analgesic treatment. Out of the nine evaluated trials, six trials demonstrated the increased risk of dyspepsia and dryness of mouth with Tapentadol use. The overall results showed Tapentadol to be a safe option with a significantly reduced risk of opioid-based adverse reactions. That said, Elderly patients need to use the medication with caution, as nausea and dizziness are more common in the elderly age group.
Tapentadol and Abuse Potential
Tapentadol has a relatively lower abuse potential in comparison to other opioids, though it can be habit-forming if used in higher doses for a prolonged period. The medication has an eighteenfold lower affinity to mu-opioid receptors than morphine, along with a lower endorsement ratio for abuse than oxymorphone.
In a sentinel sample conducted on 113,914 individuals assessed for substance abuse treatment at 624 facilities in 38 states, Tapentadol abuse was reported to be significantly less than other comparator compounds. However, it can be habit-forming or lead to overdose if taken in excess, resulting in symptoms of drowsiness, confusion, sweating, slowed breathing, and muscle weakness.
Safety and Tolerability
The majority of trials have illustrated the safety and tolerability of Tapentadol, with the medication appearing to be better tolerated than that of other opioids. Gastrointestinal events, pulmonary dysfunction, serotonin syndrome, and endocrine toxicity risks are mostly rare in Tapentadol use.
In a comparative trial of Tapentadol Prolonged Release with Oxycodone conducted by Baron et al., Tapentadol proved non-inferior to Oxycodone and had minimal impact on bowel function along with superior effectiveness. It was associated with significantly lower instances of constipation and vomiting coupled with improvements in quality of life measures.
A multicenter, open-label, Phase III trial conducted by Sabatowski et al. evaluating the effects of Tapentadol on driving ability showed that the medication did not impact driving ability, and approximately 65.7% of patients were deemed fit to drive after taking Tapentadol for severe lower back or osteoarthritis pain.

Reviews
There are no reviews yet.